Sensitive Biomarkers of PD Effects in CNS Trials
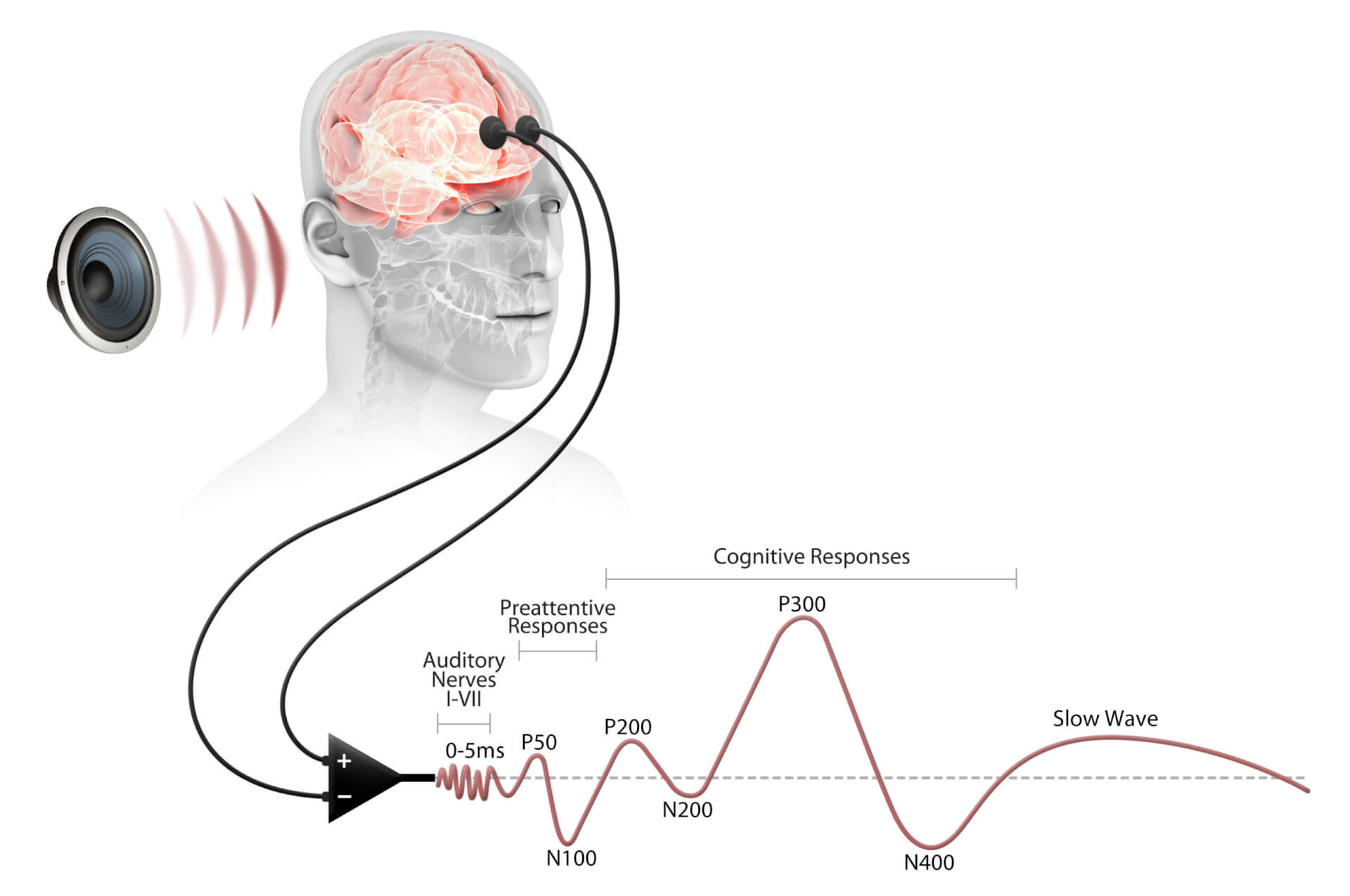
- An ERP (event-related potential) is part of the EEG produced as the brain processes a sequence of auditory stimuli.
- EEGs are recordings of the electrical activity produced by your brain, measured from the scalp.
- ERP and EEG can reliably detect small drug-induced changes in brain function.
Reliable and Scientifically Validated
- There have been over 10,000 published scientific papers using ERP measures.
- Cognision founded the ERP Biomarker Consortium consisting of 10 pharma companies to establish QEEG and ERP as a reliable biomarker in clinical trials.
- Validated in peer-reviewed papers and posters presented at major scientific conferences.
Practical & Scalable Implementation
- The COGNISION® System can be implemented at any clinical trial site with no requirement for certified EEG technicians.
- The headset is subject-friendly and takes 5 to 10 minutes of setup time.
- Suitable for testing at multiple sites with high site-to-site reliability.
- Live support available 24/7 for immediate assistance.
Advanced Functionality
- Full Clinical Trial Management System (CTMS) functionality.
- Remote monitoring of live data.
- Near real-time visibility to data & statistical results, facilitating adaptive study designs.
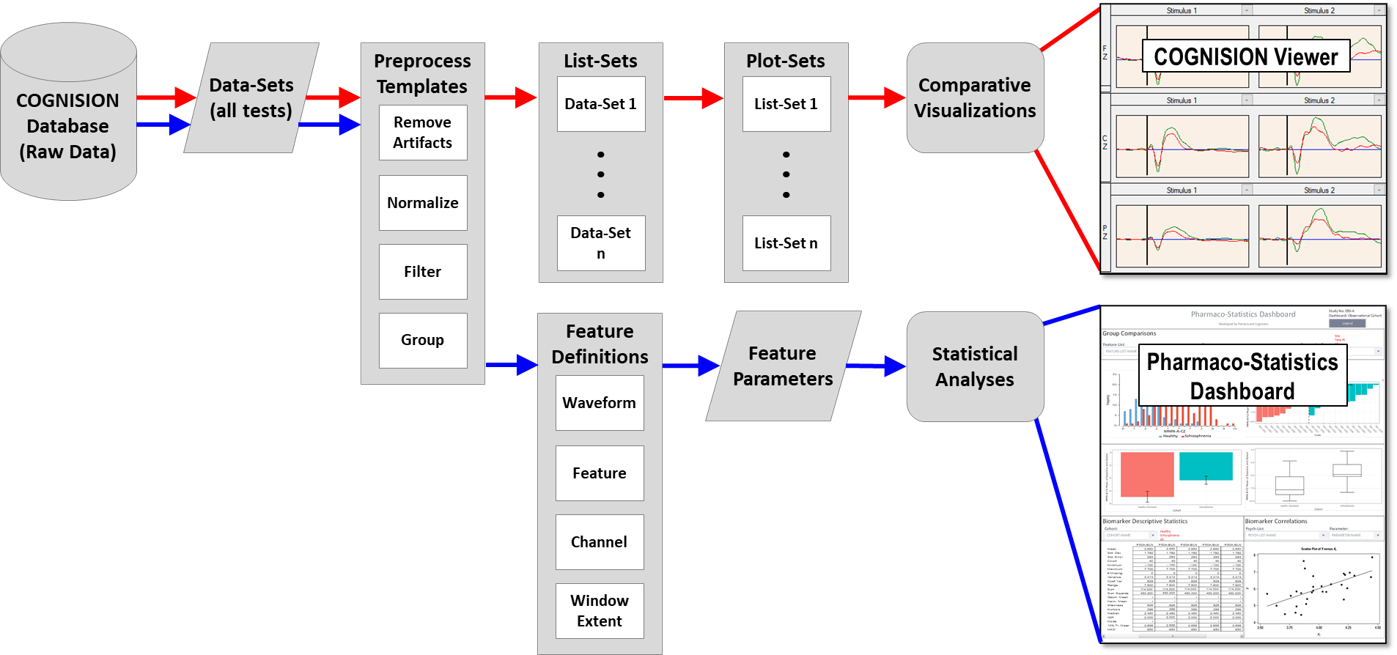
- Automated data-analysis waterfall.
- Automatic data upload to web-based database
- Includes integrated eCRF system.